Physical Properties of Haloalkanes
1. In general, haloalkanes are colourless, sweet smelling liquids. Bromides and iodides become colored when exposed to sunlight.
2. Haloalkanes are polar in nature but practically they are very slightly soluble in water. It is due to the reason that they are not able to form hydrogen bonds with water. On the other hand, chloro, bromo and iodo derivatives are soluble in organic solvents such as alcohol, ether, benzene, etc. because intermolecular forces in these derivatives are similar to that in these solvents.
3. With increasing size of the alkyl group, the densities of haloalkanes go on decreasing. Simple fluoro and chloro alkanes are lighter than water while bromides, iodides and polychloro derivatives are heavier than water. The densities increase in the order :
fluoride < chloride < bromide < iodide
4. The melting and boiling points of chlorides, bromides and iodides are considerably higher than those of the parent hydrocarbon of comparable molecular mass. It happens because the intermolecular forces of attraction (dipole-dipole and van der Waal’s) between the molecules are stronger in halogen derivatives of alkanes due to the polarity of haloalkanes.
· For the same alkyl group the boiling points of alkyl chlorides, bromides and iodides follow the order :
RI > RBr > RCl < RF where R is an alkyl group.
It is because of the reason that with the increase in the size of the halogen, the magnitude of van der Waal’s forces increases and consequently, the boiling points increase as shown below :

· In general, the boiling points of chloro, bromo and iodo compounds increases with increase in the number of halogen atoms.

· For the same halogen atom, the boiling points of haloalkanes increase with increase in the size of alkyl groups.
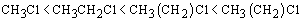
· The boiling points of isomeric alkyl halides decreases with branching. It happens due to the reason that branching of the chain makes the molecule more compact and therefore decrease the surface area. Due to decrease in surface area, the magnitude of van der Waal’s forces of attraction decreases and consequently, the boiling points of the branched chain compounds is less than those of the straight chain compounds as shown below:
n-Butylbromide > Iso-butylbromide > tert-Butyl bromide.
2. Haloalkanes are polar in nature but practically they are very slightly soluble in water. It is due to the reason that they are not able to form hydrogen bonds with water. On the other hand, chloro, bromo and iodo derivatives are soluble in organic solvents such as alcohol, ether, benzene, etc. because intermolecular forces in these derivatives are similar to that in these solvents.
3. With increasing size of the alkyl group, the densities of haloalkanes go on decreasing. Simple fluoro and chloro alkanes are lighter than water while bromides, iodides and polychloro derivatives are heavier than water. The densities increase in the order :
fluoride < chloride < bromide < iodide
4. The melting and boiling points of chlorides, bromides and iodides are considerably higher than those of the parent hydrocarbon of comparable molecular mass. It happens because the intermolecular forces of attraction (dipole-dipole and van der Waal’s) between the molecules are stronger in halogen derivatives of alkanes due to the polarity of haloalkanes.
· For the same alkyl group the boiling points of alkyl chlorides, bromides and iodides follow the order :
RI > RBr > RCl < RF where R is an alkyl group.
It is because of the reason that with the increase in the size of the halogen, the magnitude of van der Waal’s forces increases and consequently, the boiling points increase as shown below :
· In general, the boiling points of chloro, bromo and iodo compounds increases with increase in the number of halogen atoms.
· For the same halogen atom, the boiling points of haloalkanes increase with increase in the size of alkyl groups.
· The boiling points of isomeric alkyl halides decreases with branching. It happens due to the reason that branching of the chain makes the molecule more compact and therefore decrease the surface area. Due to decrease in surface area, the magnitude of van der Waal’s forces of attraction decreases and consequently, the boiling points of the branched chain compounds is less than those of the straight chain compounds as shown below:
n-Butylbromide > Iso-butylbromide > tert-Butyl bromide.

Comments
Post a Comment